To prevent child deaths, the World Health Organization (WHO) has urged member countries to take immediate action on substandard medicines.
Since October, seven countries have reported incidents of contaminated medicines containing diethylene glycol (DEG) and ethylene glycol (EG), both of which are toxic industrial chemicals.
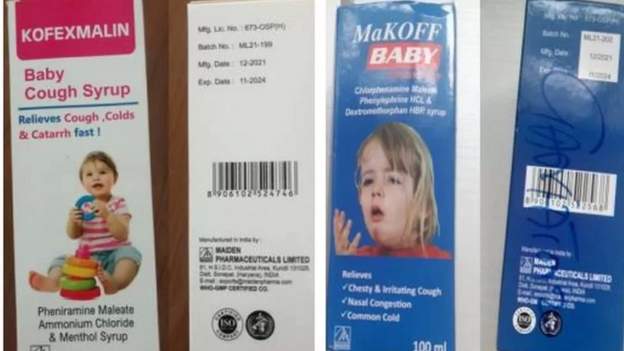
Last year, 300 children aged five and under died in The Gambia, Uzbekistan, and Indonesia as a result of substandard medicines that caused acute kidney injuries.
In all cases, the WHO issued a global alert requiring countries to withdraw the medicines from the market immediately and increase surveillance to detect them.
The global body is now urging manufacturers to purchase raw materials from qualified suppliers and test their products while keeping detailed records of the process. Distributors are also advised to sell only products that have been approved by competent authorities and to look for signs of falsification.
Poor regulatory regimes and a lack of logistics for early detection of outbreaks have frequently been blamed for the high number of fatalities. In the majority of cases, the companies involved have denied that their products are contaminated. As a result, countries have been urged to spend more resources inspecting manufacturers’ sites.
A parliamentary committee in The Gambia has recommended that Maiden Pharmaceuticals, the Indian manufacturer of cough syrups linked to the deaths of at least 70 children, be prosecuted.


